Quick Search
The Leading Quaternary Care Hospital in Abu Dhabi, UAE
Delivering Excellence in Healthcare
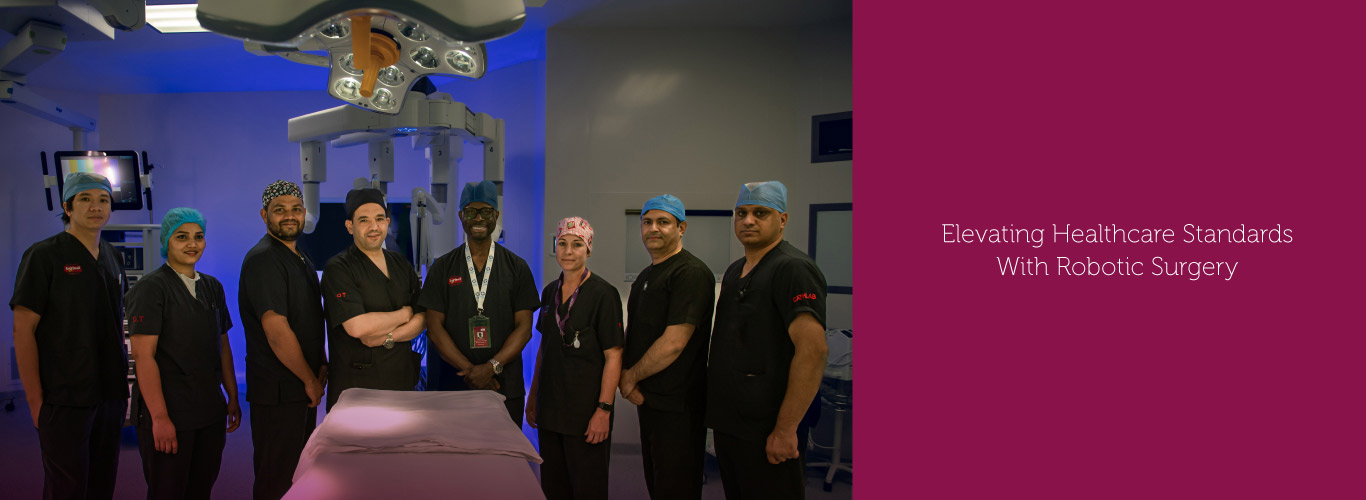
Burjeel Medical City, a flagship hospital of Burjeel Holdings, is a cutting-edge, leading quaternary care facility in Abu Dhabi, United Arab Emirates.
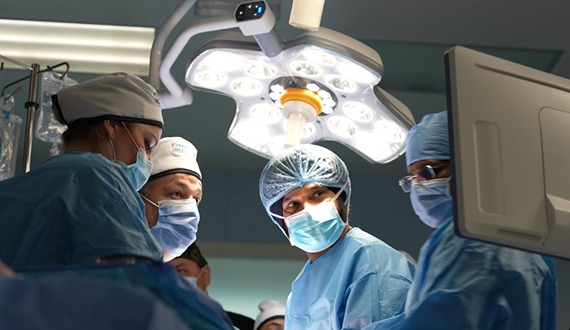
The 1.2 million-square-foot hospital houses more than 400 beds and 50+ specialties, including oncology, hematology, bone marrow transplantation, neurosurgery, multi-organ transplantation, orthopedic surgery, pediatric surgery, fetal medicine, nuclear medicine, and many others. The hospital is equipped with cutting-edge medical technology and employs Western-board certified physicians.
The hospital also houses the UAE’s most advanced rehabilitation facility, outfitted with a WalkBot, an exoskeleton gait training robot, and other cutting-edge technology. It also houses the largest hydrotherapy pool in the UAE.
The hospital’s Co-lab is a centralized diagnostic laboratory with Total Lab Automation and Infinity Lab Solutions that can run over 10 million tests annually.
Furthermore, the hospital is the first to receive ESMO accreditation as an Integrated Oncology and Palliative Care Center. The hospital’s diagnostic equipment is cutting-edge, including its first 1.5T intraoperative iMRI, 1.5 Tesla and 3 Tesla MRI, 256 slices and 128 slices of CT, PET-CT, SPECT-CT, and HOLOGIC’s 3DimensionsTM Mammography System, among many others.
The radiation oncology unit at the hospital is the most modern, with an Elekta Versa HD TM Linear Accelerator. Burjeel Medical City is the Reference Site for Elekta and Novalis Certified Radiosurgery Centers. The hospital’s multidisciplinary team of emergency physicians provides 24-hour emergency services to adult and pediatric patients. Burjeel Medical City in MBZ City is the first private hospital with a helipad for emergency medical transfers, and it provides the best treatment.
It is one of the few hospitals in the area with comprehensive pediatric subspecialties such as pediatric oncology, gastroenterology, neurology, and neonatology. Pediatric patients receive advanced care at the hospital. It also has a Level IV PICU, a Level III NICU, and Pediatric ECMO to provide the best care at the right time. Additionally, it is one of the few hospitals in the country with a specialized NICU ambulance and the only one with ECMO in in-patient and ambulatory care.